Ageing is a universal process that affects all organisms, and it occurs at multiple levels, including molecular, cellular, organ and organismal levels. The process of biological ageing is characterised by a decline in the ability of tissues and organs to regenerate and repair, leading to a decreased physiological capacity to respond to stress or homeostenosis. This process is driven by a combination of genetic, epigenetic and environmental factors and results in the progressive breakdown of complex molecular mechanisms that contribute to age-related disease.
This article is going to help you to understand about the several molecular aspects of ageing that come from research.
Ageing and its molecular biology
Ageing is a natural and complex phenomenon that involves the gradual accumulation of degeneration and damage in molecular pathways, resulting in the compromised function of cells and tissues. This process is a significant risk factor for various chronic, non-communicable diseases, i.e., diabetes, cancer, cardiovascular diseases, and neurological disorders. The mechanisms that underlie ageing and the development of these disease include DNA damage, oxidative stress, alterations in gene and non-coding RNA expression, genotoxicity and shortened telomeres. To extend human lifespan, it is essential to understand the cellular programs responsible for ageing and how their dysregulation contributes to senescence and decline. Till date, there are 14 hallmarks that explains the molecular mechanisms of ageing, including the accumulation of oxidative damage, telomere shortening, and the loss of proteostasis (1).
1. Genomic instability
Genomic instability is a common cause of ageing, resulting from the accumulation of DNA damage from both internal and external sources i.e., free radicals and radiation. While most mutations are harmless and repaired by body’s DNA repair system, a certain amount of accumulated damage occurs over time. The normal mutation rate in human DNA is approximately one mutation per billion bases per cell division, resulting in one in a million chance of a single mutation in a 1,000 base pair gene. The occurrence of genomic instability can result in small/large scale changes to DNA that disrupts protein coding sequences and regulatory elements. These alterations can contribute to a progressive decline in organ function and homeostasis. With age, there is an increased heterogeneity in gene expression among cells in the same tissue, which may lead to stochastic deregulation and contribute to the ageing process. Additionally, genetic instability can cause ageing by damaging stem cells and leading to the depletion of cells capable of division. Studies on mice cardiomyocytes have shown significant variation in gene expression among young and old individuals, with the accumulation of point mutations in small intestinal cells and large rearrangements in cardiomyocytes (2,3).
2. Telomere Shortening
Telomeres are repetitive sequences located at the ends of chromosome, which acts as protective caps against damage caused by shortening of DNA strands during replication and oxidative stress. This protection helps maintain the stability and integrity of chromosome during each round of replication. Research has shown that telomere length is inversely correlated with lifespan, meaning that individuals with shorter telomeres tend to have shorter lifespans (4).
Mammalian cell cultures enter senescence after 40–60 divisions, known as the Hayflick limit or replicative senescence (5). Shortening of the telomeres at each replication leads to cellular senescence. This telomere shortening has been implicated in many age-related phenotypes, such as decline in innate immunity and even correlates with protein diseases, such as Alzheimer’s disease (6).
3. Epigenetic alterations
Epigenetic alterations refer to changes in gene expression without altering the DNA sequence, such as DNA methylation, chromatin remodelling and non-coding DNA. There is growing evidence to suggest that epigenetic alterations play an important role in the ageing process. As we age, our cells undergo a number of changes that can lead to alterations in gene expression and ultimately, the onset of age-related diseases.
Studies have shown that DNA methylation patterns change as we age, with some genes becoming more methylated and others becoming less methylated. These changes can lead to alterations in gene expression, which in turn can contribute to age-related diseases like cancer, Alzheimer's and cardiovascular disease (7).
Histone modifications play a critical role in regulating gene expression and chromatin structure, and alterations in histone modifications have been implicated in various diseases, including cancer and ageing. In cancer, mutations in histone methyl transferases have been found to be a driver of tumor progression, and drugs targeting these enzymes are being investigated as potential cancer therapies. In ageing, specific alterations in histone modifications have been observed, such as a decrease in H3K9me3 and H3K9me2 and an increase in H3Kme3, which can contribute to changes in gene expression and chromatin structure (8).
Non-coding DNA, particularly microRNA, also play a critical role in gene regulation and are involved in the regulation of many important pathways in ageing and cancer. MicroRNA can regulate up to 80% of all expressed genes and aberrant expression of different microRNA can contribute to the development and prognosis of various cancer types. Additionally, it has been suggested that certain environmental factors, such as diet, exercise, and stress, can also affect epigenetic modifications and accelerate the ageing process (9).
4. Loss of proteostasis
Proteostasis is the maintenance of a healthy protein turnover in our bodies, which is achieved through a network of processes including chaperone-mediated folding, proteasomal degradation and autophagy. When proteostasis fails, it can result in the accumulation of misfolded and toxic protein aggregates, which is a hallmark of many age-related diseases like Alzheimer's, Parkinson's and cataracts. As we age, there is a gradual decline in proteostasis, leading to the accumulation of protein aggregates and inclusions in almost all tissues of the body. Proteostasis also plays a role in cancer development and researchers are investigating the use of drugs targeting proteostasis as potential new treatments for cancer, with some already in clinical trials. Chaperones and co-chaperones are small molecules that have been conserved throughout evolution and aid in the folding and refolding of polypeptides into functional proteins, or direct them to degradation systems if proper folding cannot be achieved (10,11).
Chaperones are molecules that help proteins fold properly and can also guide damaged proteins to be degraded. The most important type of chaperones are heat shock proteins (HSP), which are upregulated during cellular stress. As we age, our ability to induce the chaperone system in response to stress decreases, leading to an accumulation of misfolded proteins. In lab animals, overexpression of chaperones has been associated with extended lifespan (12).
5. Deregulated nutrient sensing
Nutrient sensing pathways are complex biological mechanisms that regulate cellular metabolism in response to changes in nutrient availability. These pathways include insulin and insulin-like growth factor 1 (IGF-1) signaling, mechanistic target of rapamycin (mTOR) signaling, sirtuins, and AMP-activated protein kinase (AMPK) signaling. They play critical roles in regulating energy metabolism, cell growth, and maintenance of cellular homeostasis. As we age, these nutrient-sensing pathways become less sensitive to changes in nutrient availability. This means that our cells do not respond as efficiently to changes in nutrient levels, leading to a dysregulation of metabolism. This dysregulation can contribute to the development of age-related diseases i.e., type 2 diabetes, cardiovascular disease and cancer (13).
Dysregulation of insulin and IGF-1 signaling has been linked to the development of insulin resistance, which is a hallmark of type 2 diabetes. Insulin resistance occurs when cells in the body become less responsive to the hormone insulin, which is responsible for regulating blood sugar levels. This leads to elevated blood sugar levels, which can damage tissues and organs throughout the body. Similarly, dysregulation of the mTOR pathway has been linked to the development of cancer. mTOR signaling promotes cell growth and proliferation, and excessive mTOR activity can lead to uncontrolled cell growth and tumor formation. Additionally, dysregulation of nutrient sensing pathways can impact cellular functions such as DNA repair. As we age, our cells accumulate DNA damage, which can lead to genomic instability and the development of age-related diseases. Nutrient sensing pathways play critical roles in regulating DNA repair mechanisms, and dysregulation of these pathways can impair the ability of cells to repair DNA damage (14).
6. Mitochondrial dysfunction
Mitochondria are organelles within our cells that are responsible for generating energy in the form of ATP (adenosine triphosphate) through a process called oxidative phosphorylation. As we age, mitochondrial function declines, and this dysfunction has been linked to many age-related diseases and conditions, including neurodegenerative diseases, cardiovascular disease, and type 2 diabetes.
There are several factors that are associated with mitochondrial dysfunction with ageing. One major factor is the accumulation of mutations in mitochondrial DNA (mtDNA) over time. Unlike nuclear DNA, mtDNA is not protected by histones, so it is more vulnerable to damage from oxidative stress, which can lead to mutations. Additionally, mtDNA lacks the repair mechanisms that nuclear DNA has, so these mutations can accumulate and lead to mitochondrial dysfunction.
Another factor in mitochondrial dysfunction is the accumulation of damaged or dysfunctional mitochondria within cells. Normally, cells can eliminate these dysfunctional mitochondria through a process called mitophagy, but as we age, this process becomes less efficient. This leads to a build-up of damaged mitochondria, which can further contribute to oxidative stress and damage to other cellular components.
Mitochondrial dysfunction also plays a role in the ageing process through its impact on cellular metabolism. As mitochondria produce ATP, they also generate reactive oxygen species (ROS) as a by-product. ROS can damage cellular components, including DNA, and also activate cellular signaling pathways that contribute to ageing and age-related diseases. Additionally, mitochondrial dysfunction can lead to a decrease in energy production, which can impair cellular function and contribute to age-related decline (15).
7. Cellular senescence
Cellular senescence is a process where a cell permanently stops dividing and undergoes specific changes, including alterations in the chromatin structure and secretome. This process can be triggered by numerous stress mechanisms i.e., telomere shortening, DNA damage response and oncogene activation. The tumor suppressor proteins Rb1 and p53 are critical executioners of cellular senescence. Senescence serves as an anticancer mechanism by inhibiting the proliferation of cells with telomere attrition and cells overexpressing oncogenes. However, the accumulation of senescent cells with age can be deleterious as they secrete proinflammatory cytokines and growth factors, known as the senescence-associated secretory phenotype (SASP), which has been implicated in cancer development and ageing. Inflammation is a well-known risk factor for many malignancies, and senescent cells are usually removed by immune surveillance and phagocytosis, but they seem to accumulate with age, most likely due to increased production and decreased elimination due to immunosenescence. The INK4a/ARF locus is the genetic locus most strongly linked to age-associated diseases and conditional expression of p16INK4a in mice leads to many of the physiological hallmarks of ageing. It has been found that removal of INK4a-expressing senescent cells in mice delays onset and halts the progression of ageing and associated diseases (16,17).
8. Stem cells exhaustion
Stem cells have the ability to self-renew and differentiate into various cell types, making them essential for tissue regeneration and repair throughout the lifespan. However, as we age, the function and regenerative capacity of stem cells decline. This decline is thought to contribute to the development of age-related diseases and the overall decline in tissue and organ function seen with ageing.
One of the key factors contributing to stem cell decline with age is thought to be a decrease in the number of stem cells, as well as a reduction in their regenerative capacity. For example, in the bone marrow, the number of hematopoietic stem cells (HSCs) declines with age, leading to a decreased ability to produce new blood cells. Similarly, in the brain, the number and function of neural stem cells declines with age, leading to a reduced ability to repair and regenerate damaged neurons.
In addition to changes in stem cell numbers and function, age-related changes in the stem cell microenvironment (or "niche") are also thought to play a role in stem cell decline. The stem cell niche is the microenvironment that surrounds and supports stem cells, providing them with the required signals and cues for self-renewal and differentiation. With age, changes in the niche, including changes in extracellular matrix composition, increased inflammation, and alterations in signaling molecules, can lead to a decline in stem cell function and regenerative capacity. There is evidence that suggest changes in the epigenetic landscape of stem cells may play a role in stem cell decline with age. Epigenetic changes refer to modifications to the DNA molecule that regulate gene expression, and alterations in these modifications can affect stem cell function and differentiation. For example, age-related changes in DNA methylation have been shown to affect the regenerative capacity of HSCs (18).
Overall, the decline in stem cell function and regenerative capacity with age is a complex process involving multiple factors, including changes in stem cell number, alterations in the stem cell niche, and changes in the epigenetic landscape of stem cells (19).
9. Altered intercellular communication
Cells in our body communicate with each other by releasing signaling molecules that can either affect nearby cells or travel through the bloodstream to influence cells and tissues at a distance. This communication system, known as intercellular signaling, is crucial for the proper functioning of our body. However, as we age, the process of intracellular communication becomes altered, leading to dysfunction in various cellular processes. One of the main consequences of altered intracellular communication is the development of chronic inflammation or inflammaging, which is a hallmark of ageing.
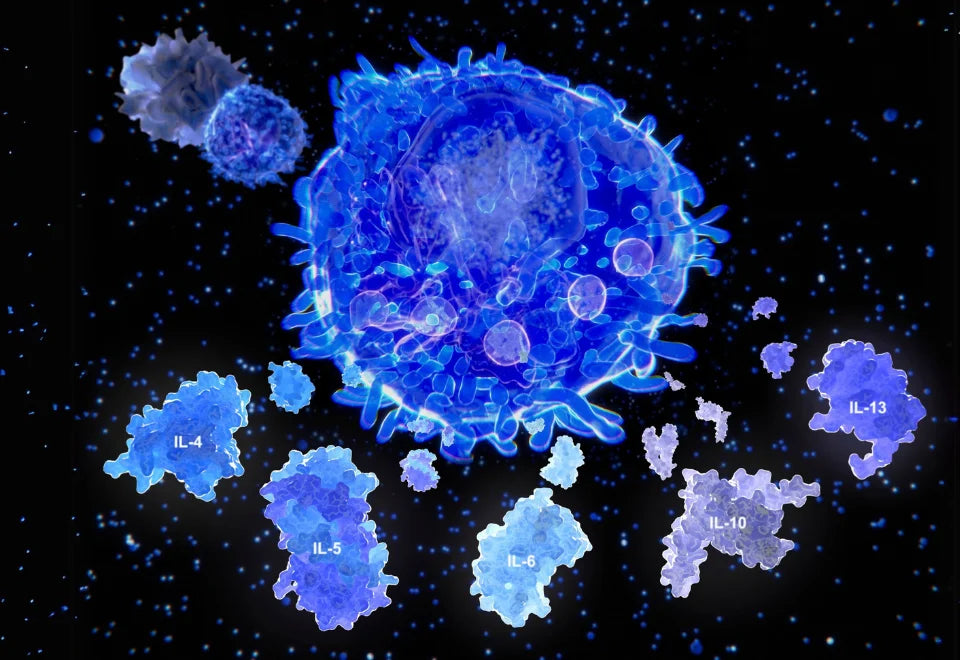
Inflammaging is characterized by the production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), which can cause tissue damage and contribute to the development of age-related diseases, such as arthritis, atherosclerosis and neurodegenerative diseases. In addition to inflammation, altered intracellular communication can also lead to impaired immune function, reduced tissue repair, and increased oxidative stress. This can result in the accumulation of damaged proteins and other cellular components, leading to cellular senescence, apoptosis, and ultimately tissue dysfunction and organ failure.
Some of the underlying mechanisms that contribute to altered intracellular communication with aging include changes in the expression of genes involved in signaling pathways, epigenetic modifications, and alterations in the composition and function of cellular membranes (20).
10. Microbiome disturbance
The microbiome is the collective term for the trillions of microorganisms that live on and inside our bodies. The microbiome plays a vital role in various physiological processes, including immune function, digestion and the production of essential vitamins and neurotransmitters. As we age, the diversity and composition of the microbiome can change, leading to dysbiosis. Dysbiosis refers to a disturbance in the balance of the microbiome, which can result in an overgrowth of harmful bacteria and a reduction in beneficial ones. This disturbance can have several negative effects on ageing.
One of the key ways that dysbiosis affects ageing is through inflammation. The microbiome plays a crucial role in regulating the immune system, and when dysbiosis occurs, the immune system can become overactive, leading to chronic inflammation and this chronic inflammation can contribute to the development of age-related diseases i.e., cardiovascular disease, type 3diabetes and neurodegenerative diseases. Dysbiosis can also affect the metabolism, which can contribute to the development of metabolic disorders such as obesity and insulin resistance. This occurs because the microbiome plays a role in regulating the absorption and storage of nutrients. Dysbiosis can result in the overgrowth of bacteria that are better at extracting energy from food, leading to increased fat storage and weight gain.
Dysbiosis can also impact ageing through the production of toxins. Certain harmful bacteria i.e., Clostridium difficile, Escherichia coli and Salmonella can produce toxins that can damage cells and contribute to the development of age-related diseases (21).
11. Compromised Autophagy
Autophagy is a cellular process that involves the breakdown and recycling of damaged or unnecessary cellular components. This process is crucial for maintaining cellular homeostasis, and dysregulation of autophagy has been implicated in the aging process. As we age, the efficacy of autophagy declines, leading to the accumulation of damaged cellular components and the accumulation of toxic proteins, which can contribute to the development of age-related diseases.
There are several mechanisms through which compromised autophagy can impact ageing. One mechanism is through the accumulation of damaged mitochondria, which can lead to increased oxidative stress and contribute to the development of age-related diseases. Autophagy also plays a key role in regulating inflammation, and compromised autophagy can lead to chronic inflammation, which has been linked to a variety of age-related diseases. Additionally, autophagy plays a role in regulating cellular senescence, which is a process by which cells enter a state of permanent growth arrest. Dysregulated autophagy can contribute to the accumulation of senescent cells, which can promote inflammation and contribute to the development of age-related diseases. Furthermore, autophagy is important for the clearance of aggregated proteins, such as amyloid-beta, which are implicated in the development of neurodegenerative diseases such as Alzheimer's (22).
12. Inflammation
Inflammation is a complex biological response to harmful stimuli i.e., pathogens, damaged cells or irritants. It involves the activation of immune cells and the release of signaling molecules i.e., cytokines and chemokines, to activate other immune cells to the site of infection or injury. Inflammation can be either acute or chronic, and while it is essential for tissue repair and healing, chronic inflammation is associated with a wide range of age-related diseases which includes cardiovascular disease, diabetes, cancer, and neurodegenerative diseases.
As we age, the immune system undergoes changes that contribute to chronic inflammation, a process also known as inflammaging. The term inflammaging refers to the low-grade, chronic inflammation that occurs with aging, even in the absence of an acute stimulus. These changes include increased production of pro-inflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha) and decreased production of anti-inflammatory cytokines, such as interleukin-10 (IL-10). This can contribute to the development of age-related diseases by promoting tissue damage, impairing tissue repair, and altering normal cellular processes, such as metabolism and cell division. Furthermore, inflammaging can also contribute to the dysregulation of the gut microbiome, leading to a vicious cycle of inflammation and dysbiosis. Dysbiosis can lead to the release of microbial products, such as lipopolysaccharides (LPS), that can stimulate the immune system and trigger inflammation. The resulting inflammation can further disrupt the gut microbiome, leading to a further increase in microbial products and inflammation. This cycle can ultimately contribute to the development of chronic diseases associated with aging, such as metabolic syndrome and neurodegenerative diseases (23).
13. Splicing dysregulation
Splicing dysregulation refers to a disruption in the normal process of RNA splicing, which is responsible for generating mature mRNA from pre-mRNA and then functional proteins. During splicing, specific segments of pre-mRNA called introns are removed, and the remaining segments called exons are joined together to form a mature mRNA molecule that can be translated into a protein.
However, when splicing is dysregulated, it can result in the inclusion or exclusion of specific exons or introns in the mature mRNA molecule. This can generate aberrant mRNA isoforms, which are mRNA molecules that contain different sequences of exons or introns than the normal, functional mRNA molecule. These aberrant mRNA isoforms may not be able to produce functional proteins or may produce proteins with altered structure or function, leading to various diseases. One possible mechanism underlying the link between splicing dysregulation and ageing is the accumulation of mutations in splicing regulatory sequences, such as exonic and intronic splicing enhancers and silencers. These mutations can alter the binding affinity of splicing factors and lead to the aberrant splicing of specific mRNA isoforms. In addition, changes in the expression or activity of splicing factors themselves, which can occur with age, may also contribute to splicing dysregulation. Splicing dysregulation can result in the production of anomalous proteins that can be toxic to cells, leading to their dysfunction or death. It can also alter the expression of key proteins involved in cellular processes, such as metabolism and DNA repair, which can contribute to the development of age-related diseases like cancer, neurodegeneration, and metabolic disorders (24).
14. Altered mechanical properties
Altered mechanical properties refers to changes in the physical properties of materials, are considered a hallmark of ageing in various types of tissues and materials, including biological tissues, metals and polymers.
In biological tissues, ageing can cause changes in the mechanical properties of tissues such as stiffness, elasticity and strength. For example, bone becomes more brittle and less flexible as it ages due to changes in its microstructure and mineral content. Similarly, in the extracellular matrix, ageing can lead to increased rigidity and loss of elasticity, making tissues less flexible and more prone to damage. These changes can also occur in cells and the extracellular environment. However, fibroblast senescence, which refers to the deterioration of fibroblast cells, can lead to alterations in cell motility and communication due to changes in the actin cytoskeleton and focal adhesions. This can cause tissue damage in the innate immune system of older individuals, leading to increased mortality rates. Additionally, ageing can cause changes in the nuclear lamina, which can destabilize the nucleoskeleton and trigger the senescence-associated secretory phenotype (SASP). The extracellular matrix, which provides structural support to cells and tissues, can also change with age, resulting in increased rigidity and loss of elasticity. These changes can contribute to age-related diseases such as hypertension and diabetes. These changes in mechanical properties are thought to be due to a variety of factors, including the accumulation of damage, changes in microstructure, and chemical reactions. For example, exposure to environmental factors such as temperature, humidity and UV radiation can cause chemical changes in polymers, affecting their mechanical properties (25).
Conclusion
The hallmarks of ageing are closely connected and occur alongside the ageing process. These hallmarks can be categorized into primary, antagonistic and integrative categories. Primary hallmarks, such as genomic instability and telomere attrition are the initiators of cellular damage, while antagonistic hallmarks, including mitochondrial dysfunction and deregulated nutrient sensing, can be either beneficial or harmful depending on their levels. Integrative hallmarks result from the accumulation of damage from primary and antagonistic hallmarks and directly interfere with tissue homeostasis.
Defining the hallmarks of ageing is important for understanding the molecular mechanisms of ageing and designing interventions to improve human health-span. By understanding these hallmarks, we can potentially develop strategies to slow down or reverse the ageing process, thereby extending healthy lifespan.
However, there are challenges in understanding this complex process. Next-generation sequencing technologies can help evaluate genetic and epigenetic changes in ageing cells. These techniques are already being used to study exceptional longevity in humans, compare animal species, and analyse age-related epigenetic changes at high resolution.


























Leave a comment
All comments are moderated before being published.
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.